Search
- Page Path
- HOME > Search
- Thyroid
- Active Surveillance for Low-Risk Thyroid Cancers: A Review of Current Practice Guidelines
- Min Joo Kim, Jae Hoon Moon, Eun Kyung Lee, Young Shin Song, Kyong Yeun Jung, Ji Ye Lee, Ji-hoon Kim, Kyungsik Kim, Sue K. Park, Young Joo Park
- Endocrinol Metab. 2024;39(1):47-60. Published online February 15, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1937
- 1,913 View
- 173 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
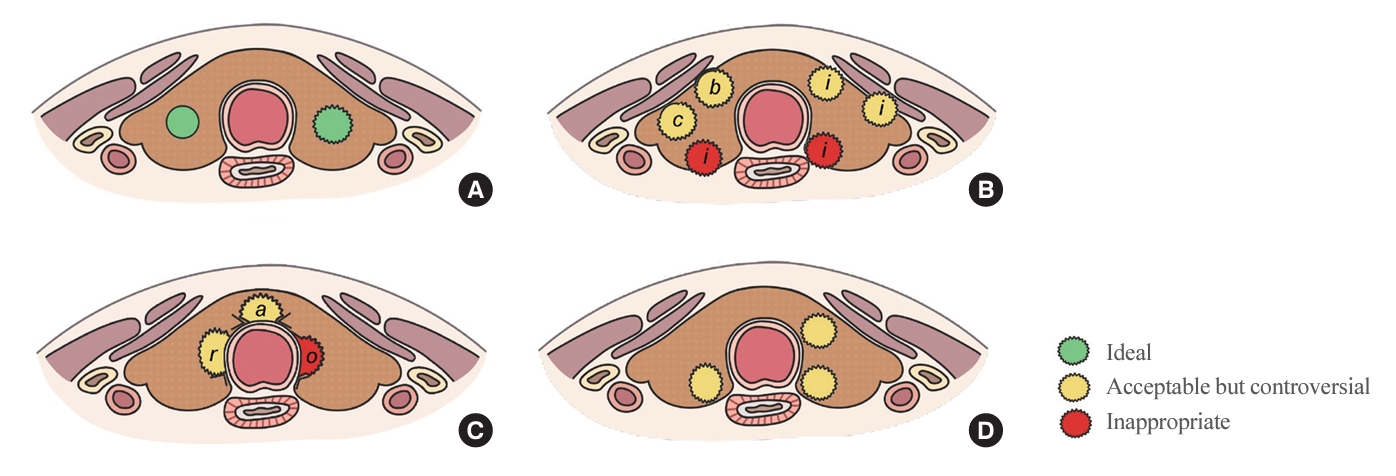
ePub - The indolent nature and favorable outcomes associated with papillary thyroid microcarcinoma have prompted numerous prospective studies on active surveillance (AS) and its adoption as an alternative to immediate surgery in managing low-risk thyroid cancer. This article reviews the current status of AS, as outlined in various international practice guidelines. AS is typically recommended for tumors that measure 1 cm or less in diameter and do not exhibit aggressive subtypes on cytology, extrathyroidal extension, lymph node metastasis, or distant metastasis. To determine the most appropriate candidates for AS, factors such as tumor size, location, multiplicity, and ultrasound findings are considered, along with patient characteristics like medical condition, age, and family history. Moreover, shared decision-making, which includes patient-reported outcomes such as quality of life and cost-effectiveness, is essential. During AS, patients undergo regular ultrasound examinations to monitor for signs of disease progression, including tumor growth, extrathyroidal extension, or lymph node metastasis. In conclusion, while AS is a feasible and reliable approach for managing lowrisk thyroid cancer, it requires careful patient selection, effective communication for shared decision-making, standardized follow-up protocols, and a clear definition of disease progression.

- Thyroid
- A Narrative Review of the 2023 Korean Thyroid Association Management Guideline for Patients with Thyroid Nodules
- Eun Kyung Lee, Young Joo Park, Chan Kwon Jung, Dong Gyu Na
- Endocrinol Metab. 2024;39(1):61-72. Published online February 14, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1938
- 1,517 View
- 98 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
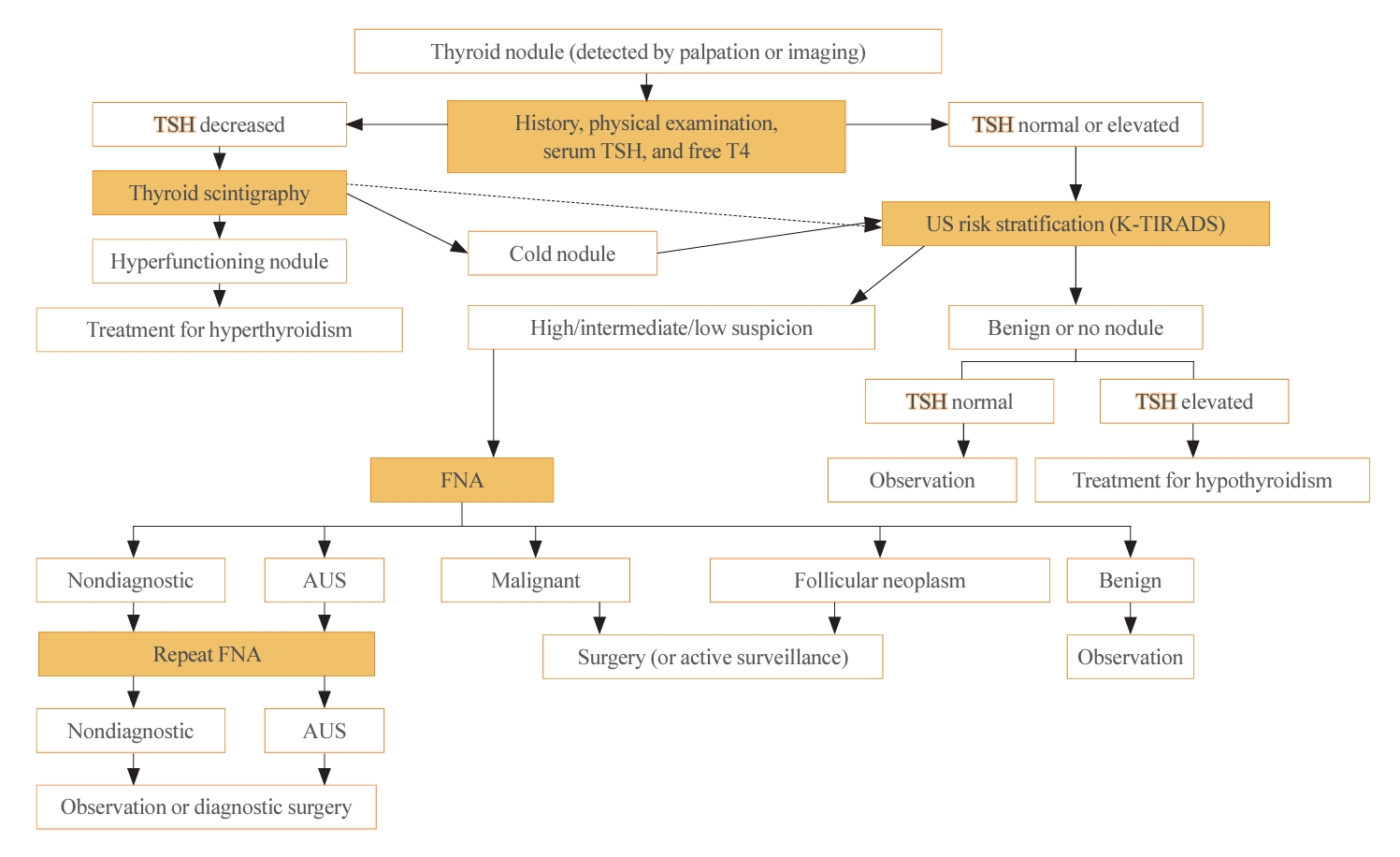
ePub - The 2023 Korean Thyroid Association (KTA) Management Guideline for Patients with Thyroid Nodules constitute an update of the 2016 KTA guideline for thyroid nodules and cancers that focuses specifically on nodules. The 2023 guideline aim to offer updated guidance based on new evidence that reflects the changes in clinical practice since the 2016 KTA guideline. To update the 2023 guideline, a comprehensive literature search was conducted from January 2022 to May 2022. The literature search included studies, reviews, and other evidence involving human subjects that were published in English in MEDLINE (PubMed), Embase, and other relevant databases. Additional significant clinical trials and research studies published up to April 2023 were also reviewed. The limitations of the current evidence are discussed, and suggestions for areas in need of further research are identified. The purpose of this review is to provide a summary of the 2023 KTA guideline for the management of thyroid nodules released in May 2023 and to give a balanced insight with comparison of recent guidelines from other societies.
-
Citations
Citations to this article as recorded by- 2023 Update of the Korean Thyroid Association Guidelines for the Management of Thyroid Nodules
Eun Kyung Lee, Young Joo Park
Clinical Thyroidology®.2024; 36(4): 153. CrossRef
- 2023 Update of the Korean Thyroid Association Guidelines for the Management of Thyroid Nodules

- Miscellaneous
- Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
- Won Sang Yoo, Eu Jeong Ku, Eun Kyung Lee, Hwa Young Ahn
- Endocrinol Metab. 2023;38(6):750-759. Published online November 13, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1785
- 1,446 View
- 122 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigated the incidence of endocrine immune-related adverse events (irAEs) for recently developed immune checkpoint inhibitor (ICI) drugs.
Methods
We collected studies on newly developed ICI drugs using PubMed/Medline, Embase, and Cochrane Library from inception through January 31, 2023. Among ICI drugs, nivolumab, pembrolizumab, and ipilimumab were excluded from the new ICI drugs because many papers on endocrine-related side effects have already been published.
Results
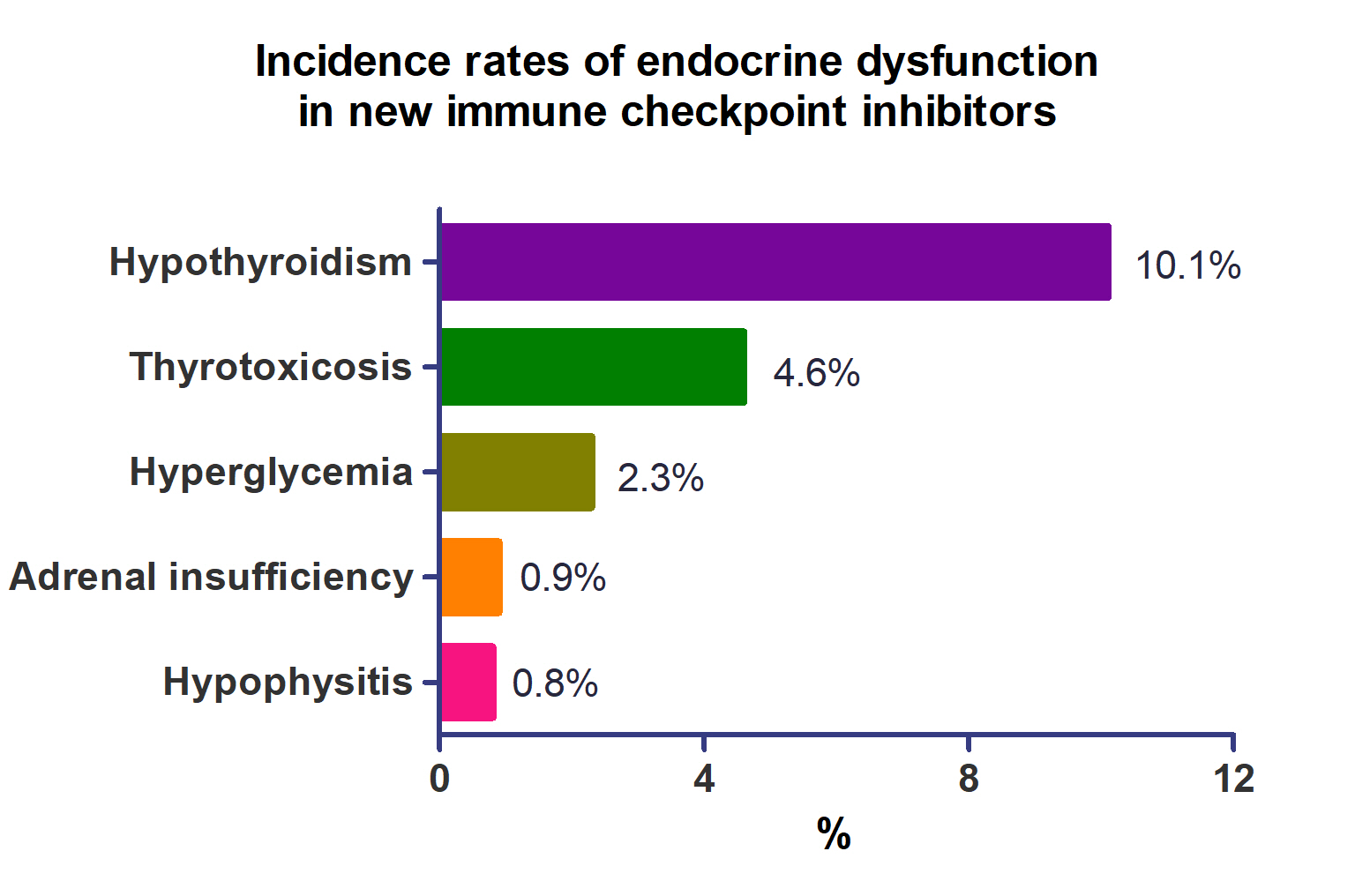
A total of 44,595 patients from 177 studies were included in this analysis. The incidence of hypothyroidism was 10.1% (95% confidence interval [CI], 8.9% to 11.4%), thyrotoxicosis was 4.6% (95% CI, 3.8% to 5.7%), hypophysitis was 0.8% (95% CI, 0.5% to 1.1%), adrenal insufficiency was 0.9% (95% CI, 0.7% to 1.1%), and hyperglycemia was 2.3% (95% CI, 1.6% to 3.4%). Hypothyroidism and thyrotoxicosis occurred most frequently with programmed cell death protein-1 (PD-1) inhibitors (13.7% and 7.5%, respectively). The rate of endocrine side effects for the combination of a programmed death-ligand 1 inhibitor (durvalumab) and cytotoxic T lymphocyte-associated antigen 4 inhibitor (tremelimumab) was higher than that of monotherapy. In a meta-analysis, the combination of tremelimumab and durvalumab had a 9- to 10-fold higher risk of pituitary and adrenal-related side effects than durvalumab alone.
Conclusion
Newly developed PD-1 inhibitors had a high incidence of thyroid-related irAEs, and combined treatment with durvalumab and tremelimumab increased the risk of pituitary- and adrenal-related irAEs. Based on these facts, it is necessary to predict the endocrine side effects corresponding to each ICI drug, diagnose and treat them appropriately, and try to reduce the morbidity and mortality of patients.

- Thyroid
Thyroid Cancer Screening - Survival Comparison of Incidentally Found versus Clinically Detected Thyroid Cancers: An Analysis of a Nationwide Cohort Study
- Shinje Moon, Eun Kyung Lee, Hoonsung Choi, Sue K. Park, Young Joo Park
- Endocrinol Metab. 2023;38(1):81-92. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1668
- 1,738 View
- 154 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The true benefit of thyroid cancer screening is incompletely understood. This study investigated the impact of ultrasound screening on thyroid cancer outcomes through a comparison with symptomatic thyroid cancer using data from a nationwide cohort study in Korea.
Methods
Cox regression analysis was performed to assess the hazard ratios (HRs) for all-cause and thyroid cancer-specific mortality. Considering the possible bias arising from age, sex, year of thyroid cancer registration, and confounding factors for mortality (including smoking/drinking status, diabetes, and hypertension), all analyses were conducted with stabilized inverse probability of treatment weighting (IPTW) according to the route of detection.
Results
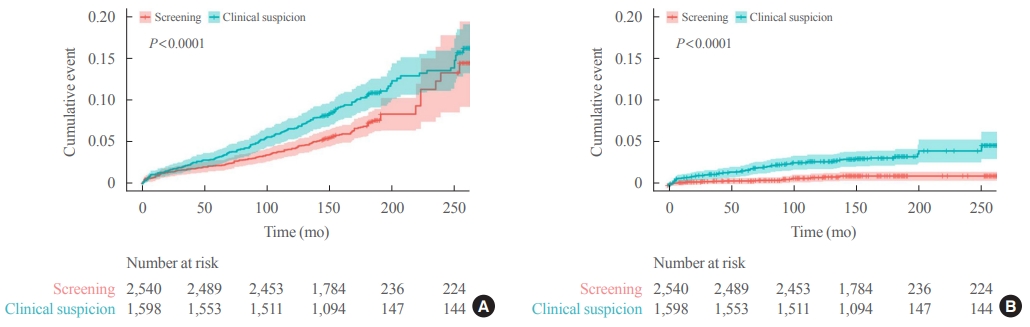
Of 5,796 patients with thyroid cancer, 4,145 were included and 1,651 were excluded due to insufficient data. In comparison with the screening group, the clinical suspicion group was associated with large tumors (17.2±14.6 mm vs. 10.4±7.9 mm), advanced T stage (3–4) (odds ratio [OR], 1.24; 95% confidence interval [CI], 1.09 to 1.41), extrathyroidal extension (OR, 1.16; 95% CI, 1.02 to 1.32), and advanced stage (III–IV) (OR, 1.16; 95% CI, 1.00 to 1.35). In IPTW-adjusted Cox regression analysis, the clinical suspicion group had significantly higher risks of all-cause mortality (HR, 1.43; 95% CI, 1.14 to 1.80) and thyroid cancer-specific mortality (HR, 3.07; 95% CI, 1.77 to 5.29). Mediation analysis showed that the presence of thyroid-specific symptoms was directly associated with a higher risk of cancer-specific mortality. Thyroid-specific symptoms also indirectly affected thyroid cancer-specific mortality, mediated by tumor size and advanced clinicopathologic status.
Conclusion
Our findings provide important evidence for the survival benefit of early detection of thyroid cancer compared to symptomatic thyroid cancer. -
Citations
Citations to this article as recorded by- Cost-Utility Analysis of Early Detection with Ultrasonography of Differentiated Thyroid Cancer: A Retrospective Study on a Korean Population
Han-Sang Baek, Jeonghoon Ha, Kwangsoon Kim, Ja Seong Bae, Jeong Soo Kim, Sungju Kim, Dong-Jun Lim, Chul-Min Kim
Endocrinology and Metabolism.2024; 39(2): 310. CrossRef - Clinical Characteristics, Diagnostic Approach and Outcome of Thyroid Incidental Findings vs. Clinically Overt Thyroid Nodules: An Observational Single-Centre Study
Tom Jansen, Nike Stikkelbroeck, Annenienke van de Ven, Ilse van Engen-van Grunsven, Marcel Janssen, Han Bonenkamp, Martin Gotthardt, Romana T. Netea-Maier
Cancers.2023; 15(8): 2350. CrossRef - Lower Thyroid Cancer Mortality in Patients Detected by Screening: A Meta-Analysis
Shinje Moon, Young Shin Song, Kyong Yeun Jung, Eun Kyung Lee, Young Joo Park
Endocrinology and Metabolism.2023; 38(1): 93. CrossRef - To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - The 2017 United States Preventive Services Task Force Recommendation for Thyroid Cancer Screening Is No Longer the Gold Standard
Ka Hee Yi
Endocrinology and Metabolism.2023; 38(1): 72. CrossRef - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinology and Metabolism.2023; 38(1): 75. CrossRef
- Cost-Utility Analysis of Early Detection with Ultrasonography of Differentiated Thyroid Cancer: A Retrospective Study on a Korean Population

- Thyroid
Thyroid Cancer Screening - Lower Thyroid Cancer Mortality in Patients Detected by Screening: A Meta-Analysis
- Shinje Moon, Young Shin Song, Kyong Yeun Jung, Eun Kyung Lee, Young Joo Park
- Endocrinol Metab. 2023;38(1):93-103. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1667

- 2,188 View
- 117 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Thyroid cancer screening has contributed to the skyrocketing prevalence of thyroid cancer. However, the true benefit of thyroid cancer screening is not fully understood. This study aimed to evaluate the impact of screening on the clinical outcomes of thyroid cancer by comparing incidental thyroid cancer (ITC) with non-incidental thyroid cancer (NITC) through a meta-analysis.
Methods
PubMed and Embase were searched from inception to September 2022. We estimated and compared the prevalence of high-risk features (aggressive histology of thyroid cancer, extrathyroidal extension, metastasis to regional lymph nodes or distant organs, and advanced tumor-node-metastasis [TNM] stage), thyroid cancer-specific death, and recurrence in the ITC and NITC groups. We also calculated pooled risks and 95% confidence intervals (CIs) of the outcomes derived from these two groups.
Results
From 1,078 studies screened, 14 were included. In comparison to NITC, the ITC group had a lower incidence of aggressive histology (odds ratio [OR], 0.46; 95% CI, 0.31 to 0.7), smaller tumors (mean difference, −7.9 mm; 95% CI, −10.2 to −5.6), lymph node metastasis (OR, 0.64; 95% CI, 0.48 to 0.86), and distant metastasis (OR, 0.42; 95% CI, 0.23 to 0.77). The risks of recurrence and thyroid cancer-specific mortality were also lower in the ITC group (OR, 0.42; 95% CI, 0.25 to 0.71 and OR, 0.46; 95% CI, 0.28 to 0.74) than in the NITC group.
Conclusion
Our findings provide important evidence of a survival benefit from the early detection of thyroid cancer compared to symptomatic thyroid cancer. -
Citations
Citations to this article as recorded by- To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - The 2017 United States Preventive Services Task Force Recommendation for Thyroid Cancer Screening Is No Longer the Gold Standard
Ka Hee Yi
Endocrinology and Metabolism.2023; 38(1): 72. CrossRef - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinology and Metabolism.2023; 38(1): 75. CrossRef - Delayed Surgery for and Outcomes of Papillary Thyroid Cancer: Is the Pendulum Still Swinging?
Giorgio Grani
Clinical Thyroidology.2023; 35(5): 192. CrossRef
- To Screen or Not to Screen?

- Diabetes, Obesity and Metabolism
- You Can’t Avoid Shift Work? Then Focus on Body Fat Rather than Weight
- Eun Kyung Lee
- Endocrinol Metab. 2022;37(5):756-758. Published online October 25, 2022
- DOI: https://doi.org/10.3803/EnM.2022.501
- 1,283 View
- 131 Download

- Thyroid
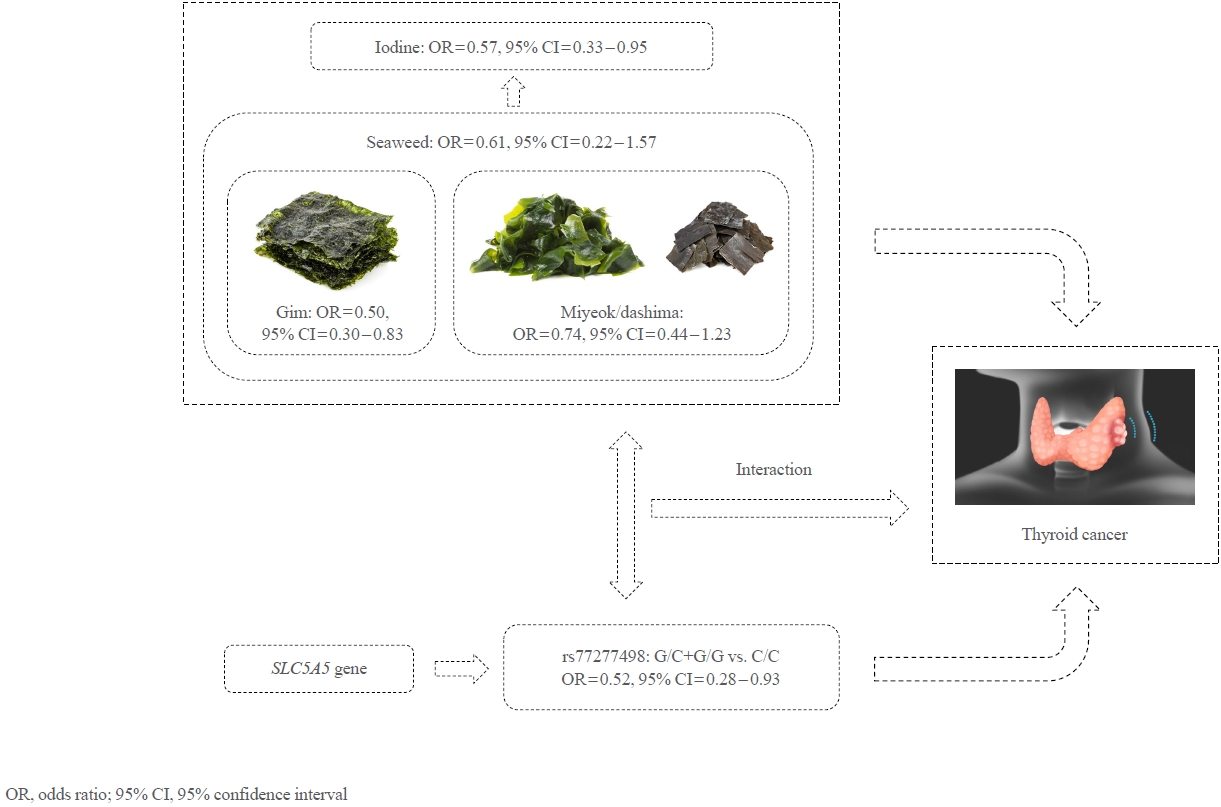
- Seaweed and Iodine Intakes and SLC5A5 rs77277498 in Relation to Thyroid Cancer
- Tung Hoang, Eun Kyung Lee, Jeonghee Lee, Yul Hwangbo, Jeongseon Kim
- Endocrinol Metab. 2022;37(3):513-523. Published online May 24, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1306
- 3,354 View
- 140 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study aims to elucidate the associations among dietary seaweed (gim and miyeok/dashima) and iodine intakes, the rs77277498 polymorphism of the SLC5A5 gene codifying the sodium/iodine symporter, and thyroid cancer risk in a Korean population.
Methods
We conducted a case-control study of 117 thyroid cancer cases and 173 controls who participated in the Cancer Screenee Cohort between 2002 and 2014 at the National Cancer Center, Korea. The amount of seaweed and iodine consumption (g/day) was estimated using the residual energy adjustment method. We calculated odds ratios (ORs) and their 95% confidence intervals (CIs) using a multivariable logistic regression model for the separate and combined effect of dietary iodine-based intake and SLC5A5 polymorphism (rs77277498, C>G) on thyroid cancer.
Results
Dietary gim and iodine intakes were inversely associated with thyroid cancer, with ORs of 0.50 (95% CI, 0.30 to 0.83) and 0.57 (95% CI, 0.35 to 0.95), respectively, whereas the associations for dietary miyeok/dashima and total seaweed intakes were not significant. However, compared with individuals carrying the C/C genotype of the rs77277498 polymorphism with a low intake of all dietary factors, those carrying the G allele with a high intake had a lower risk of thyroid cancer, with ORs of 0.25 (95% CI, 0.10 to 0.56), 0.31 (95% CI, 0.12 to 0.77), 0.26 (95% CI, 0.10 to 0.62), and 0.30 (95% CI, 0.12 to 0.73) for the consumption of gim, miyeok/dashima, total seaweed, and iodine, respectively.
Conclusion
In summary, our results supported the evidence of the protective effects of dietary gim and iodine intake against thyroid cancer risk, and this association can be strengthened by SLC5A5 rs77277498 genotypes. -
Citations
Citations to this article as recorded by- Iodine nutrition and papillary thyroid cancer
Xueqi Zhang, Fan Zhang, Qiuxian Li, Chuyao Feng, Weiping Teng
Frontiers in Nutrition.2022;[Epub] CrossRef
- Iodine nutrition and papillary thyroid cancer

- Thyroid
- A Multicenter, Randomized, Controlled Trial for Assessing the Usefulness of Suppressing Thyroid Stimulating Hormone Target Levels after Thyroid Lobectomy in Low to Intermediate Risk Thyroid Cancer Patients (MASTER): A Study Protocol
- Eun Kyung Lee, Yea Eun Kang, Young Joo Park, Bon Seok Koo, Ki-Wook Chung, Eu Jeong Ku, Ho-Ryun Won, Won Sang Yoo, Eonju Jeon, Se Hyun Paek, Yong Sang Lee, Dong Mee Lim, Yong Joon Suh, Ha Kyoung Park, Hyo-Jeong Kim, Bo Hyun Kim, Mijin Kim, Sun Wook Kim, Ka Hee Yi, Sue K. Park, Eun-Jae Jung, June Young Choi, Ja Seong Bae, Joon Hwa Hong, Kee-Hyun Nam, Young Ki Lee, Hyeong Won Yu, Sujeong Go, Young Mi Kang, MASTER study group
- Endocrinol Metab. 2021;36(3):574-581. Published online May 26, 2021
- DOI: https://doi.org/10.3803/EnM.2020.943
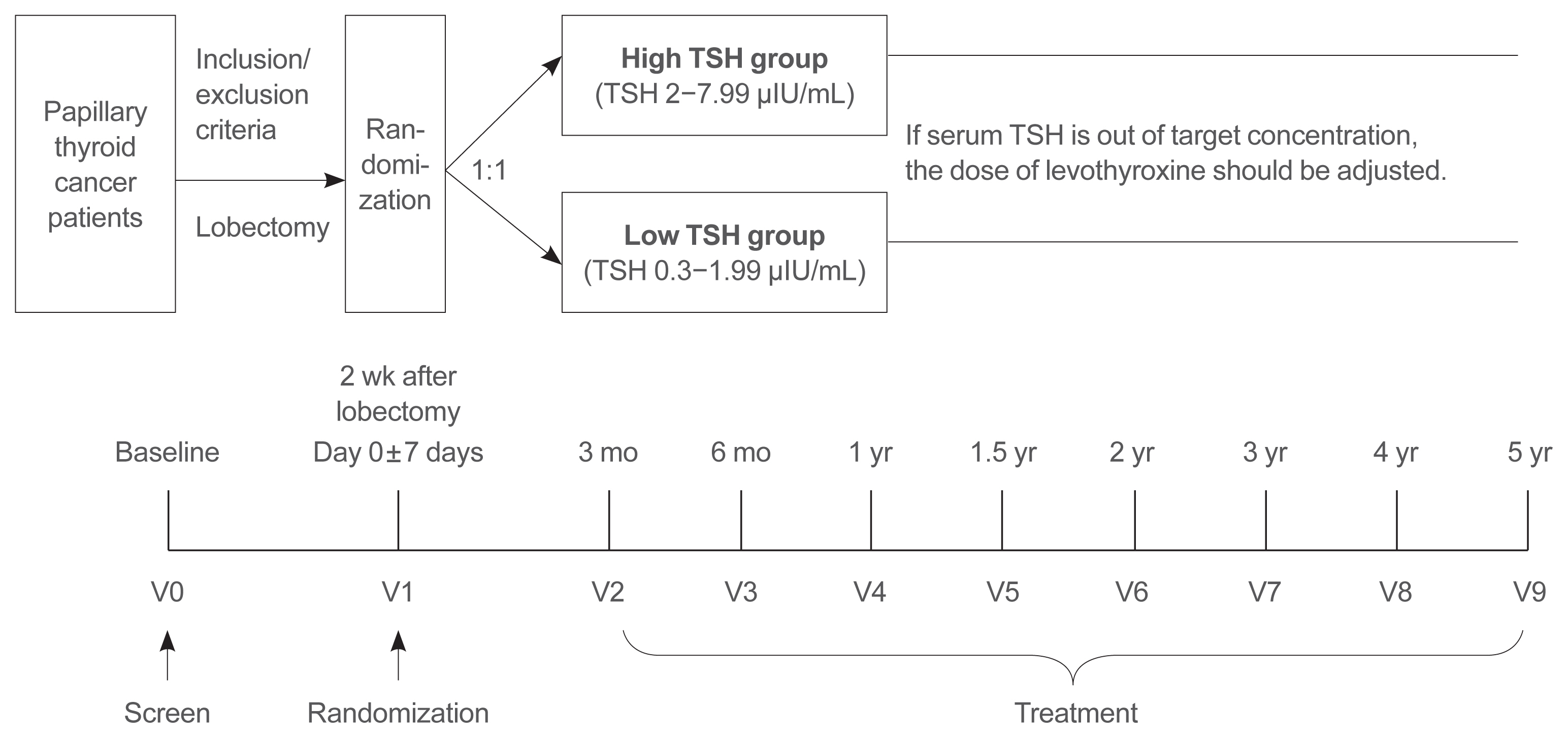
- 6,299 View
- 268 Download
- 8 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Postoperative thyroid stimulating hormone (TSH) suppression therapy is recommended for patients with intermediate- and high-risk differentiated thyroid cancer to prevent the recurrence of thyroid cancer. With the recent increase in small thyroid cancer cases, the extent of resection during surgery has generally decreased. Therefore, questions have been raised about the efficacy and long-term side effects of TSH suppression therapy in patients who have undergone a lobectomy.
Methods
This is a multicenter, prospective, randomized, controlled clinical trial in which 2,986 patients with papillary thyroid cancer are randomized into a high-TSH group (intervention) and a low-TSH group (control) after having undergone a lobectomy. The principle of treatment includes a TSH-lowering regimen aimed at TSH levels between 0.3 and 1.99 μIU/mL in the low-TSH group. The high-TSH group targets TSH levels between 2.0 and 7.99 μIU/mL. The dose of levothyroxine will be adjusted at each visit to maintain the target TSH level. The primary outcome is recurrence-free survival, as assessed by neck ultrasound every 6 to 12 months. Secondary endpoints include disease-free survival, overall survival, success rate in reaching the TSH target range, the proportion of patients with major cardiovascular diseases or bone metabolic disease, the quality of life, and medical costs. The follow-up period is 5 years.
Conclusion
The results of this trial will contribute to establishing the optimal indication for TSH suppression therapy in low-risk papillary thyroid cancer patients by evaluating the benefit and harm of lowering TSH levels in terms of recurrence, metabolic complications, costs, and quality of life. -
Citations
Citations to this article as recorded by- Effect of thyroid-stimulating hormone suppression on quality of life in thyroid lobectomy patients: interim analysis of a multicenter, randomized controlled trial in low- to intermediate-risk thyroid cancer patients (MASTER study)
Ja Kyung Lee, Eu Jeong Ku, Su-jin Kim, Woochul Kim, Jae Won Cho, Kyong Yeun Jung, Hyeong Won Yu, Yea Eun Kang, Mijin Kim, Hee Kyung Kim, Junsun Ryu, June Young Choi
Annals of Surgical Treatment and Research.2024; 106(1): 19. CrossRef - Clinical impact of coexistent chronic lymphocytic thyroiditis on central lymph node metastasis in low- to intermediate-risk papillary thyroid carcinoma: The MASTER study
Da Beom Heo, Ho-Ryun Won, Kyung Tae, Yea Eun Kang, Eonju Jeon, Yong Bae Ji, Jae Won Chang, June Young Choi, Hyeong Won Yu, Eu Jeong Ku, Eun Kyung Lee, Mijin Kim, Jun-Ho Choe, Bon Seok Koo
Surgery.2024; 175(4): 1049. CrossRef - Dynamic Changes in Treatment Response af-ter 131I in Differentiated Thyroid Cancer and Their Relationship with Recurrence Risk Stratification and TNM Staging
璐 狄
Advances in Clinical Medicine.2024; 14(03): 1083. CrossRef - ASO Author Reflections: Active Surveillance may be Possible in Patients with T1b Papillary Thyroid Carcinoma Over 55 Years of Age Without High-Risk Features on Preoperative Examinations
Ho-Ryun Won, Eonju Jeon, Da Beom Heo, Jae Won Chang, Minho Shong, Je Ryong Kim, Hyemi Ko, Yea Eun Kang, Hyon-Seung Yi, Ju Hee Lee, Kyong Hye Joung, Ji Min Kim, Younju Lee, Sung-Woo Kim, Young Ju Jeong, Yong Bae Ji, Kyung Tae, Bon Seok Koo
Annals of Surgical Oncology.2023; 30(4): 2254. CrossRef - Outcomes and Trends of Treatments in High‐Risk Differentiated Thyroid Cancer
Arash Abiri, Khodayar Goshtasbi, Sina J. Torabi, Edward C. Kuan, William B. Armstrong, Tjoson Tjoa, Yarah M. Haidar
Otolaryngology–Head and Neck Surgery.2023; 168(4): 745. CrossRef - Current Controversies in Low-Risk Differentiated Thyroid Cancer: Reducing Overtreatment in an Era of Overdiagnosis
Timothy M Ullmann, Maria Papaleontiou, Julie Ann Sosa
The Journal of Clinical Endocrinology & Metabolism.2023; 108(2): 271. CrossRef - Age-Dependent Clinicopathological Characteristics of Patients with T1b Papillary Thyroid Carcinoma: Implications for the Possibility of Active Surveillance
Ho-Ryun Won, Eonju Jeon, Da Beom Heo, Jae Won Chang, Minho Shong, Je Ryong Kim, Hyemi Ko, Yea Eun Kang, Hyon-Seung Yi, Ju Hee Lee, Kyong Hye Joung, Ji Min Kim, Younju Lee, Sung-Woo Kim, Young Ju Jeong, Yong Bae Ji, Kyung Tae, Bon Seok Koo
Annals of Surgical Oncology.2023; 30(4): 2246. CrossRef - Potential impact of obesity on the aggressiveness of low- to intermediate-risk papillary thyroid carcinoma: results from a MASTER cohort study
Mijin Kim, Yae Eun Kang, Young Joo Park, Bon Seok Koo, Eu Jeong Ku, June Young Choi, Eun Kyung Lee, Bo Hyun Kim
Endocrine.2023; 82(1): 134. CrossRef - Differentiated thyroid cancer: a focus on post-operative thyroid hormone replacement and thyrotropin suppression therapy
Benjamin J. Gigliotti, Sina Jasim
Endocrine.2023; 83(2): 251. CrossRef - Thyroid stimulating hormone suppression and recurrence after thyroid lobectomy for papillary thyroid carcinoma
Mi Rye Bae, Sung Hoon Nam, Jong-Lyel Roh, Seung-Ho Choi, Soon Yuhl Nam, Sang Yoon Kim
Endocrine.2022; 75(2): 487. CrossRef - The Concept of Economic Evaluation and Its Application in Thyroid Cancer Research
Kyungsik Kim, Mijin Kim, Woojin Lim, Bo Hyun Kim, Sue K. Park
Endocrinology and Metabolism.2021; 36(4): 725. CrossRef
- Effect of thyroid-stimulating hormone suppression on quality of life in thyroid lobectomy patients: interim analysis of a multicenter, randomized controlled trial in low- to intermediate-risk thyroid cancer patients (MASTER study)

- Thyroid
- Best Achievements in Clinical Thyroidology in 2020
- Eun Kyung Lee, Young Joo Park
- Endocrinol Metab. 2021;36(1):30-35. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2021.103
- 4,453 View
- 246 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - This review highlights the most interesting research in thyroidology conducted in 2020. The publications of interest discussed below dealt with the following topics: thyroid dysfunction, risk of thyroid cancer, molecular diagnostics and new therapeutics for thyroid cancer, and thyroid disease in the coronavirus disease 2019 pandemic era.
-
Citations
Citations to this article as recorded by- Compensation for iodine deficiency conditions with drugs based on duckweed substrate
M. Kh. Sadulaev, M. I. Usmanova, T. T. Tataev, A. M. Inderbiev, A. S.-A. Zhamalullayla, A. Salamova
BIO Web of Conferences.2023; 76: 03002. CrossRef - Use of long non-coding RNAs for the molecular diagnosis of papillary thyroid cancer
Daham Kim, Juyeon Yu, Jiwon Kim, Yoon-a Hwang, Jin Kyong Kim, Cheol Ryong Ku, Jung Hyun Yoon, Jin Young Kwak, Kee-Hyun Nam, Eun Jig Lee
Frontiers in Oncology.2022;[Epub] CrossRef - Ultrasound-Guided Fine-Needle Aspiration with or without Negative Pressure for Different Types of Thyroid Nodules
Qi Zhou, Wenjun Wu, Fang Wang, Xiaohua Gong, Xiaojun Chen
International Journal of General Medicine.2021; Volume 14: 5475. CrossRef
- Compensation for iodine deficiency conditions with drugs based on duckweed substrate

- Clinical Study
- A Phase II Multi-Center, Non-Randomized, Parallel Group, Non-Inferiority Study to Compare the Efficacy of No Radioactive Iodine Remnant Ablation to Remnant Ablation Treatment in Low- to Intermediate-Risk of Papillary Thyroid Cancer: The MOREthyroid Trial Protocol
- Eun Kyung Lee, You Jin Lee, Young Joo Park, Jae Hoon Moon, Ka Hee Yi, Koon Soon Kim, Joo Hee Lee, Sun Wook Cho, Jungnam Joo, Yul Hwangbo, Sujeong Go, Do Joon Park
- Endocrinol Metab. 2020;35(3):571-577. Published online September 22, 2020
- DOI: https://doi.org/10.3803/EnM.2020.681
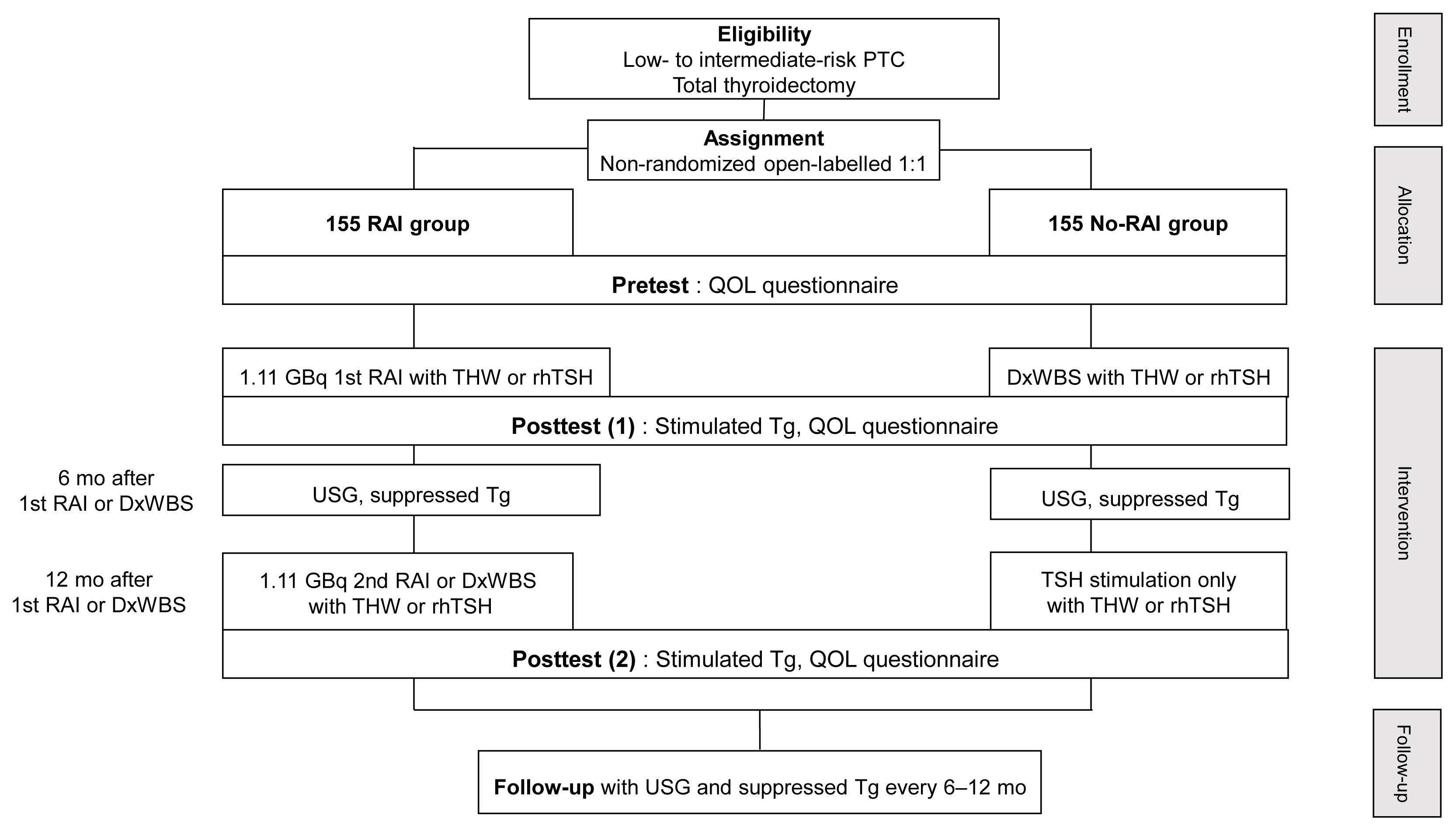
- 4,640 View
- 119 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Radioactive iodine (RAI) remnant ablation is recommended in patients with papillary thyroid cancer (PTC) and extrathyroidal extension or central lymph node metastasis. However, there exists little evidence about the necessity of remnant ablation in PTC patients with low- to intermediate-risk, those have been increasing in recent decades.
Methods
This multicenter, prospective, non-randomized, parallel group clinical trial will enroll 310 eligible patients with low- to intermediate-risk of thyroid cancer. Inclusion criteria are patients who recently underwent total thyroidectomy for PTC with 3 or less tumors of size 1≤ to ≤2 cm with no microscopic extension and N0/x, or size ≤2 cm with microscopic extension and/or N1a (number of lymph node ≤3, size of tumor foci ≤0.2 cm, and lymph node ratio <0.4). Patients choose to undergo RAI ablation (131I, dose 1.1 GBq) or diagnostic whole-body scan (DxWBS) (131I or 123I, dose 0.074 to 0.222 GBq), followed by subsequent measurement of stimulated thyroglobulin (sTg) within 1 year. Survey for quality of life (QOL) will be performed at baseline and at 1 year after follow-up. The total enrollment period is 5 years, and patients will be followed up for 1 year. The primary endpoint is the non-inferiority of surgery alone to surgery with ablation in terms of biochemical remission (BCR) rate (sTg ≤2 ng/mL) without evidence of structural recurrence. The secondary endpoint was the difference of QOL.
Conclusion
This study will evaluate whether surgery alone achieves similar BCR and improved QOL compared to RAI ablation in patients with low- to intermediate-risk PTC within 1 year.

- Thyroid
- Natural Killer Cells and Thyroid Diseases
- Eun Kyung Lee, John B. Sunwoo
- Endocrinol Metab. 2019;34(2):132-137. Published online June 24, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.2.132
- 5,952 View
- 92 Download
- 15 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Abnormal production of thyroid hormone is one of the common endocrine disorders, and thyroid hormone production declines with age. The aging process also negatively affects the immune system. An interaction between endocrine system and the immune system has been proposed to be bidirectional. Emerging evidence suggests an interaction between a lymphocyte population, called natural killer (NK) cells and thyroid gland function. Here, we review the relationship between NK cells and thyroid function and disease.
-
Citations
Citations to this article as recorded by- Tumor microenvironment in thyroid cancer: Immune cells, patterns, and novel treatments
Beatriz Febrero, Juan José Ruiz‐Manzanera, Inmaculada Ros‐Madrid, Antonio Miguel Hernández, Esteban Orenes‐Piñero, José Manuel Rodríguez
Head & Neck.2024;[Epub] CrossRef - Thyroid hormones and minerals in immunocorrection of disorders in autoimmune thyroid diseases
Viktor Kravchenko, Tamara Zakharchenko
Frontiers in Endocrinology.2023;[Epub] CrossRef - New Insights into Immune Cells and Immunotherapy for Thyroid Cancer
Yujia Tao, Peng Li, Chao Feng, Yuan Cao
Immunological Investigations.2023; 52(8): 1039. CrossRef - Innate Immunity in Autoimmune Thyroid Disease during Pregnancy
Tatjana Bogović Crnčić, Neva Girotto, Maja Ilić Tomaš, Ines Krištofić, Sanja Klobučar, Lara Batičić, Božena Ćurko-Cofek, Vlatka Sotošek
International Journal of Molecular Sciences.2023; 24(20): 15442. CrossRef - Risk Factors Associated with Mortality among Patients with COVID-19: Analysis of a Cohort of 1213 Patients in a Tertiary Healthcare Center
Carlos Alfonso Romero-Gameros, Guadalupe Vargas-Ortega, Mario Enrique Rendón-Macias, Carlos Fredy Cuevas-García, Tania Colín-Martínez, Luis Alejandro Sánchez-Hurtado, Lourdes Josefina Balcázar-Hernández, Iván Emilio De la Cruz-Rodríguez, Enid Karina Pérez
Journal of Clinical Medicine.2022; 11(10): 2780. CrossRef - Combined unsupervised and semi-automated supervised analysis of flow cytometry data reveals cellular fingerprint associated with newly diagnosed pediatric type 1 diabetes
Camillo Bechi Genzano, Eugenia Bezzecchi, Debora Carnovale, Alessandra Mandelli, Elisa Morotti, Valeria Castorani, Valeria Favalli, Angela Stabilini, Vittoria Insalaco, Francesca Ragogna, Valentina Codazzi, Giulia Maria Scotti, Stefania Del Rosso, Benedet
Frontiers in Immunology.2022;[Epub] CrossRef - Long COVID and the Neuroendocrinology of Microbial Translocation Outside the GI Tract: Some Treatment Strategies
Adonis Sfera, Carolina Osorio, Sabine Hazan, Zisis Kozlakidis, Jose Campo Maldonado, Carlos Manuel Zapata-Martín del Campo, Jonathan J. Anton, Leah Rahman, Christina V. Andronescu, Garth L. Nicolson
Endocrines.2022; 3(4): 703. CrossRef - HS3ST3A1 and CAPN8 Serve as Immune-Related Biomarkers for Predicting the Prognosis in Thyroid Cancer
Zhao-Hui Chen, Hao-Ran Yue, Jun-Hui Li, Ruo-Yu Jiang, Xiao-Ning Wang, Xue-Jie Zhou, Yue Yu, Xu-Chen Cao, Rengyun Liu
Journal of Oncology.2022; 2022: 1. CrossRef - Construction of an Expression Classifier Based on an Immune-related Ten-gene Panel for Rapid Diagnosis of Papillary Thyroid Carcinoma Risks
Jingxue Sun, Jingjing Li, Yaguang Zhang, Jun Han, Jiaxing Wei, Yanmeizhi Wu, Bing Liu, Hongyu Han, Hong Qiao
Current Bioinformatics.2022; 17(10): 924. CrossRef - Papillary Thyroid Carcinoma Landscape and Its Immunological Link With Hashimoto Thyroiditis at Single-Cell Resolution
Jun Pan, Fang Ye, Chengxuan Yu, Qinsheng Zhu, Jiaqi Li, Yaohui Zhang, Hedi Tian, Yunjin Yao, Minjie Zhu, Yibin Shen, Feng Zhu, Yingying Wang, Xinhui Zhou, Guoji Guo, Yijun Wu
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - The Intriguing Thyroid Hormones–Lung Cancer Association as Exemplification of the Thyroid Hormones–Cancer Association: Three Decades of Evolving Research
Maria V. Deligiorgi, Dimitrios T. Trafalis
International Journal of Molecular Sciences.2021; 23(1): 436. CrossRef - Mass Cytometry Studies of Patients With Autoimmune Endocrine Diseases Reveal Distinct Disease-Specific Alterations in Immune Cell Subsets
Louise Magnusson, Hugo Barcenilla, Mikael Pihl, Sophie Bensing, Daniel Espes, Per-Ola Carlsson, Rosaura Casas
Frontiers in Immunology.2020;[Epub] CrossRef - Reduced proportion and activity of natural killer cells in patients with Graves’ disease
Qingqing Yang, Li Zhang, Cheng Guo, ChunJia Kou, Yu Long, Jianting Li, Hai-Qing Zhang
European Journal of Inflammation.2020; 18: 205873922094233. CrossRef - Immunological Drivers in Graves' Disease: NK Cells as a Master Switcher
Daniela Gallo, Eliana Piantanida, Matteo Gallazzi, Luigi Bartalena, Maria Laura Tanda, Antonino Bruno, Lorenzo Mortara
Frontiers in Endocrinology.2020;[Epub] CrossRef - Quantitative and Functional Analysis of PD-1+ NK Cells in Patients With Autoimmune Thyroid Disease
Alma Cesleste Ortega-Rodríguez, Rebeca Martínez-Hernández, Adriana Monsiváis-Urenda, Ana Serrano-Somavilla, Raquel Sánchez-Gutiérrez, Roberto González-Amaro, Mónica Marazuela
The Journal of Clinical Endocrinology & Metabolism.2020; 105(11): e4001. CrossRef - Immune Cell Confrontation in the Papillary Thyroid Carcinoma Microenvironment
Zhenyu Xie, Xin Li, Yuzhen He, Song Wu, Shiyue Wang, Jianjian Sun, Yuchen He, Yu Lun, Jian Zhang
Frontiers in Endocrinology.2020;[Epub] CrossRef - Immune and Inflammatory Cells in Thyroid Cancer Microenvironment
Ferrari, Fallahi, Galdiero, Ruffilli, Elia, Ragusa, Paparo, Patrizio, Mazzi, Varricchi, Marone, Antonelli
International Journal of Molecular Sciences.2019; 20(18): 4413. CrossRef
- Tumor microenvironment in thyroid cancer: Immune cells, patterns, and novel treatments

- Miscellaneous
- Corrigendum: Author's Name Correction. Study Protocol of Multicenter Prospective Cohort Study of Active Surveillance on Papillary Thyroid Microcarcinoma (MAeSTro)
- Jae Hoon Moon, Ji-hoon Kim, Eun Kyung Lee, Kyu Eun Lee, Sung Hye Kong, Yeo Koon Kim, Woo-Jin Jeong, Chang Yoon Lee, Roh-Eul Yoo, Yul Hwangbo, Young Shin Song, Min Joo Kim, Sun Wook Cho, Su-jin Kim, Eun-Jae Chung, June Young Choi, Chang Hwan Ryu, You Jin Lee, Jeong Hun Hah, Yuh-Seog Jung, Junsun Ryu, Yunji Hwang, Sue K. Park, Ho Kyung Sung, Ka Hee Yi, Do Joon Park, Young Joo Park
- Endocrinol Metab. 2018;33(3):427. Published online August 14, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.3.427
- 3,510 View
- 48 Download
- 2 Web of Science
- 2 Crossref
-
 PDF
PDF PubReader
PubReader  ePub
ePub -
Citations
Citations to this article as recorded by- Invasiveness and Metastatic Aggressiveness in Small Differentiated Thyroid Cancers: Demography of Small Papillary Thyroid Carcinomas in the Swedish Population
Haytham Bayadsi, Martin Bergman, Malin Sund, Joakim Hennings
World Journal of Surgery.2020; 44(2): 461. CrossRef - Clinical and pathologic predictors of lymph node metastasis in papillary thyroid microcarcinomas
Ling Zhao, Xiaoya Sun, Yukun Luo, Fulin Wang, Zhaohui Lyu
Annals of Diagnostic Pathology.2020; 49: 151647. CrossRef
- Invasiveness and Metastatic Aggressiveness in Small Differentiated Thyroid Cancers: Demography of Small Papillary Thyroid Carcinomas in the Swedish Population

- Thyroid
- Study Protocol of Multicenter Prospective Cohort Study of Active Surveillance on Papillary Thyroid Microcarcinoma (MAeSTro)
- Jae Hoon Moon, Ji-hoon Kim, Eun Kyung Lee, Kyu Eun Lee, Sung Hye Kong, Yeo Koon Kim, Woo-jin Jung, Chang Yoon Lee, Roh-Eul Yoo, Yul Hwangbo, Young Shin Song, Min Joo Kim, Sun Wook Cho, Su-jin Kim, Eun Jae Jung, June Young Choi, Chang Hwan Ryu, You Jin Lee, Jeong Hun Hah, Yuh-Seog Jung, Junsun Ryu, Yunji Hwang, Sue K. Park, Ho Kyung Sung, Ka Hee Yi, Do Joon Park, Young Joo Park
- Endocrinol Metab. 2018;33(2):278-286. Published online June 21, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.2.278
- 5,448 View
- 88 Download
- 34 Web of Science
- 32 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background The ongoing Multicenter Prospective Cohort Study of Active Surveillance on Papillary Thyroid Microcarcinoma (MAeSTro) aims to observe the natural course of papillary thyroid microcarcinoma (PTMC), develop a protocol for active surveillance (AS), and compare the long-term prognosis, quality of life, and medical costs between the AS and immediate surgery groups.
Methods This multicenter prospective cohort study of PTMC started in June 2016. The inclusion criteria were suspicious of malignancy or malignancy based on fine needle aspiration or core needle biopsy, age of ≥18 years, and a maximum diameter of ≤1 cm. If there was no major organ involvement, no lymph node/distant metastasis, and no variants with poor prognosis, the patients were explained of the pros and cons of immediate surgery and AS before selecting AS or immediate surgery. Follow-up visits (physical examination, ultrasonography, thyroid function, and questionnaires) are scheduled every 6 months during the first 2 years, and then every 1 year thereafter. Progression was defined as a maximum diameter increase of ≥3, ≥2 mm in two dimensions, suspected organ involvement, or lymph node/distant metastasis.
Results Among 439 enrolled patients, 290 patients (66.1%) chose AS and 149 patients (33.9%) chose immediate surgery. The median follow-up was 6.7 months (range, 0.2 to 11.9). The immediate surgery group had a larger maximum tumor diameter, compared to the AS group (7.1±1.9 mm vs. 6.6±2.0 mm, respectively;
P =0.014).Conclusion The results will be useful for developing an appropriate PTMC treatment policy based on its natural course and risk factors for progression.
-
Citations
Citations to this article as recorded by- 2023 Update of the Korean Thyroid Association Guidelines for the Management of Thyroid Nodules
Eun Kyung Lee, Young Joo Park
Clinical Thyroidology®.2024; 36(4): 153. CrossRef - Psychological adjustment to initial treatment for low‐risk thyroid cancer: Preliminary study
Gabriella T. Seo, Mark L. Urken, Lauren E. Wein, Michael P. Saturno, Danielle Kapustin, Monica H. Xing, Lauren E. Yue, Eric M. Dowling, Tracey A. Revenson, Katherine J. Roberts, Robert Michael Tuttle
Head & Neck.2023; 45(2): 439. CrossRef - Response to Letter to the Editor on Surgical Outcomes in Patients With Low-Risk Papillary Thyroid Microcarcinoma From MAeSTro Study: Immediate Operation Versus Delayed Operation Following Active Surveillance: A Multicenter Prospective Cohort Study
Hyeonuk Hwang, June Young Choi, Jae Hoon Moon, Eun Kyung Lee, Young Joo Park, Su-jin Kim, Yuh-Seog Jung
Annals of Surgery Open.2023; 4(3): e311. CrossRef - Lower Thyroid Cancer Mortality in Patients Detected by Screening: A Meta-Analysis
Shinje Moon, Young Shin Song, Kyong Yeun Jung, Eun Kyung Lee, Young Joo Park
Endocrinology and Metabolism.2023; 38(1): 93. CrossRef - To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - Surgical Outcomes in Patients With Low-risk Papillary Thyroid Microcarcinoma From MAeSTro Study
Hyeonuk Hwang, June Young Choi, Hyeong Won Yu, Jae Hoon Moon, Ji-hoon Kim, Eun Kyung Lee, Yeo Koon Kim, Chang Yoon Lee, Sun Wook Cho, Eun-Jae Chung, Chang Hwan Ryu, Junsun Ryu, Ka Hee Yi, Do Joon Park, Kyu Eun Lee, Young Joo Park, Su-jin Kim, Yuh-Seog Jun
Annals of Surgery.2023; 278(5): e1087. CrossRef - US Predictors of Papillary Thyroid Microcarcinoma Progression at Active Surveillance
Ji Ye Lee, Ji-hoon Kim, Yeo Koon Kim, Chang Yoon Lee, Eun Kyung Lee, Jae Hoon Moon, Hoon Sung Choi, Hwangbo Yul, Sun Wook Cho, Su-jin Kim, Kyu Eun Lee, Do Joon Park, Young Joo Park
Radiology.2023;[Epub] CrossRef - MET-receptor targeted fluorescent imaging and spectroscopy to detect multifocal papillary thyroid cancer
Madelon J. H. Metman, Pascal K. C. Jonker, Luc H. J. Sondorp, Bettien M. van Hemel, Mark S. Sywak, Anthony J. Gill, Liesbeth Jansen, Paul J. van Diest, Tessa M. van Ginhoven, Clemens W. G. M. Löwik, Anh H. Nguyen, Dominic J. Robinson, Gooitzen M. van Dam,
European Journal of Nuclear Medicine and Molecular Imaging.2023;[Epub] CrossRef - Active Surveillance of Thyroid Microcarcinomas: a Critical View
Claudio R. Cernea, Leandro Luongo Matos, Cecília Eugênio, Giovanna Mattos Ferreira, Yasmin Sa Cerqueira, Ana Kober N. Leite, Felipe A. B. Vanderlei, Dorival de Carlucci, Renato N. Gotoda, Flávio C. Hojaij, Vergilius J. F. Araújo-Filho
Current Oncology Reports.2022; 24(1): 69. CrossRef - Active Surveillance Versus Thyroid Surgery for Differentiated Thyroid Cancer: A Systematic Review
Roger Chou, Tracy Dana, Megan Haymart, Angela M. Leung, Ralph P. Tufano, Julie Ann Sosa, Matthew D. Ringel
Thyroid.2022; 32(4): 351. CrossRef - A Review of Active Surveillance of Papillary Thyroid Microcarcinoma
Cho Rok Lee
Journal of Endocrine Surgery.2022; 22(1): 1. CrossRef - Active Surveillance Versus Immediate Surgery for Low-Risk Papillary Thyroid Microcarcinoma Patients in South Korea: A Cost-Minimization Analysis from the MAeSTro Study
Kyungsik Kim, June Young Choi, Su-jin Kim, Eun Kyung Lee, Young Ki Lee, Jun Sun Ryu, Kyu Eun Lee, Jae Hoon Moon, Young Joo Park, Sun Wook Cho, Sue K. Park
Thyroid.2022; 32(6): 648. CrossRef - A cross-sectional survey of patient treatment choice in a multicenter prospective cohort study on active surveillance of papillary thyroid microcarcinoma (MAeSTro)
Yul Hwangbo, June Young Choi, Eun Kyung Lee, Chang Hwan Ryu, Sun Wook Cho, Eun Jae Chung, Jeong Hun Hah, Woo-Jin Jeong, Sue K. Park, Yuh-Seog Jung, Ji-hoon Kim, Min Joo Kim, Su-jin Kim, Yeo Koon Kim, Chang Yoon Lee, Ji Ye Lee, You Jin Lee, Hyeong Won Yu,
Thyroid.2022;[Epub] CrossRef - Progression of Low-Risk Papillary Thyroid Microcarcinoma During Active Surveillance: Interim Analysis of a Multicenter Prospective Cohort Study of Active Surveillance on Papillary Thyroid Microcarcinoma in Korea
Eun Kyung Lee, Jae Hoon Moon, Yul Hwangbo, Chang Hwan Ryu, Sun Wook Cho, June Young Choi, Eun-Jae Chung, Woo-Jin Jeong, Yuh-Seog Jung, Junsun Ryu, Su-jin Kim, Min Joo Kim, Yeo Koon Kim, Chang Yoon Lee, Ji Ye Lee, Hyeong Won Yu, Jeong Hun Hah, Kyu Eun Lee,
Thyroid.2022; 32(11): 1328. CrossRef - Indications and Strategy for Active Surveillance of Adult Low-Risk Papillary Thyroid Microcarcinoma: Consensus Statements from the Japan Association of Endocrine Surgery Task Force on Management for Papillary Thyroid Microcarcinoma
Iwao Sugitani, Yasuhiro Ito, Dai Takeuchi, Hirotaka Nakayama, Chie Masaki, Hisakazu Shindo, Masanori Teshima, Kazuhiko Horiguchi, Yusaku Yoshida, Toshiharu Kanai, Mitsuyoshi Hirokawa, Kiyomi Y. Hames, Isao Tabei, Akira Miyauchi
Thyroid.2021; 31(2): 183. CrossRef - Effect of Initial Treatment Choice on 2-year Quality of Life in Patients with Low-risk Papillary Thyroid Microcarcinoma
Jae Hoon Moon, Chang Hwan Ryu, Sun Wook Cho, June Young Choi, Eun-Jae Chung, Jeong Hun Hah, Yul Hwangbo, Woo-Jin Jeong, Yuh-Seog Jung, Ji-hoon Kim, Min Joo Kim, Su-jin Kim, Yeo Koon Kim, Chang Yoon Lee, Eun Kyung Lee, Ji Ye Lee, Kyu Eun Lee, You Jin Lee,
The Journal of Clinical Endocrinology & Metabolism.2021; 106(3): 724. CrossRef - Adoption of Active Surveillance for Very Low-Risk Differentiated Thyroid Cancer in the United States: A National Survey
Susan C Pitt, Nan Yang, Megan C Saucke, Nicholas Marka, Bret Hanlon, Kristin L Long, Alexandria D McDow, J P Brito, Benjamin R Roman
The Journal of Clinical Endocrinology & Metabolism.2021; 106(4): 1728. CrossRef - Protocol for a Korean Multicenter Prospective Cohort Study of Active Surveillance or Surgery (KoMPASS) in Papillary Thyroid Microcarcinoma
Min Ji Jeon, Yea Eun Kang, Jae Hoon Moon, Dong Jun Lim, Chang Yoon Lee, Yong Sang Lee, Sun Wook Kim, Min-Hee Kim, Bo Hyun Kim, Ho-Cheol Kang, Minho Shong, Sun Wook Cho, Won Bae Kim
Endocrinology and Metabolism.2021; 36(2): 359. CrossRef - Multifocality and Progression of Papillary Thyroid Microcarcinoma During Active Surveillance
Ryuta Nagaoka, Aya Ebina, Kazuhisa Toda, Tomoo Jikuzono, Marie Saitou, Masaomi Sen, Hiroko Kazusaka, Mami Matsui, Keiko Yamada, Hiroki Mitani, Iwao Sugitani
World Journal of Surgery.2021; 45(9): 2769. CrossRef - Active Surveillance as an Effective Management Option for Low-Risk Papillary Thyroid Microcarcinoma
Min Ji Jeon, Won Gu Kim, Tae Yong Kim, Young Kee Shong, Won Bae Kim
Endocrinology and Metabolism.2021; 36(4): 717. CrossRef - The Concept of Economic Evaluation and Its Application in Thyroid Cancer Research
Kyungsik Kim, Mijin Kim, Woojin Lim, Bo Hyun Kim, Sue K. Park
Endocrinology and Metabolism.2021; 36(4): 725. CrossRef - Genomic and Transcriptomic Characteristics According to Size of Papillary Thyroid Microcarcinoma
Young Shin Song, Byung-Hee Kang, Seungbok Lee, Seong-Keun Yoo, Young Sik Choi, Jungsun Park, Dong Yoon Park, Kyu Eun Lee, Jeong-Sun Seo, Young Joo Park
Cancers.2020; 12(5): 1345. CrossRef - Experience with Active Surveillance of Thyroid Low-Risk Carcinoma in a Developing Country
Alvaro Sanabria
Thyroid.2020; 30(7): 985. CrossRef - Association of Patient Age With Progression of Low-risk Papillary Thyroid Carcinoma Under Active Surveillance
Alexandra Koshkina, Rouhi Fazelzad, Iwao Sugitani, Akira Miyauchi, Lehana Thabane, David P. Goldstein, Sangeet Ghai, Anna M. Sawka
JAMA Otolaryngology–Head & Neck Surgery.2020; 146(6): 552. CrossRef - Active surveillance in low risk papillary thyroid carcinoma
Fabian Pitoia, Anabella Smulever
World Journal of Clinical Oncology.2020; 11(6): 320. CrossRef - Early Diagnosis of Low-Risk Papillary Thyroid Cancer Results Rather in Overtreatment Than a Better Survival
Jolanta Krajewska, Aleksandra Kukulska, Malgorzata Oczko-Wojciechowska, Agnieszka Kotecka-Blicharz, Katarzyna Drosik-Rutowicz, Malgorzata Haras-Gil, Barbara Jarzab, Daria Handkiewicz-Junak
Frontiers in Endocrinology.2020;[Epub] CrossRef - The dilemma of papillary thyroid microcarcinoma management. To operate or not to operate, that is the question
Juan C Galofré
Endocrinología, Diabetes y Nutrición.2019; 66(8): 469. CrossRef - Computed Tomography for Detecting Cervical Lymph Node Metastasis in Patients Who Have Papillary Thyroid Microcarcinoma with Tumor Characteristics Appropriate for Active Surveillance
Dong-Hwa Lee, Yeo Koon Kim, Hyeong Won Yu, June Young Choi, So Yeon Park, Jae Hoon Moon
Thyroid.2019; 29(11): 1653. CrossRef - The dilemma of papillary thyroid microcarcinoma management. To operate or not to operate, that is the question
Juan C Galofré
Endocrinología, Diabetes y Nutrición (English ed.).2019; 66(8): 469. CrossRef - Longitudinal Assessment of Quality of Life According to Treatment Options in Low-Risk Papillary Thyroid Microcarcinoma Patients: Active Surveillance or Immediate Surgery (Interim Analysis of MAeSTro)
Sung Hye Kong, Junsun Ryu, Min Joo Kim, Sun Wook Cho, Young Shin Song, Ka Hee Yi, Do Joon Park, Yul Hwangbo, You Jin Lee, Kyu Eun Lee, Su-jin Kim, Woo-Jin Jeong, Eun-Jae Chung, Jeong Hun Hah, June Young Choi, Chang Hwan Ryu, Yuh-Seog Jung, Jae Hoon Moon,
Thyroid.2019; 29(8): 1089. CrossRef - Ultrasound-guided percutaneous laser ablation for papillary thyroid microcarcinoma: a retrospective analysis of 37 patients
Lili Ji, Qin Wu, Jun Gu, Xuedong Deng, Wei Zhou, Xing Fan, Feng Zhou
Cancer Imaging.2019;[Epub] CrossRef - Evolving management considerations in active surveillance for micropapillary thyroid carcinoma
Allen S. Ho, Irene Chen, Michelle Melany, Wendy L. Sacks
Current Opinion in Endocrinology, Diabetes & Obesity.2018; 25(5): 353. CrossRef
- 2023 Update of the Korean Thyroid Association Guidelines for the Management of Thyroid Nodules

- Acute Hyperglycemia Associated with Anti-Cancer Medication
- Yul Hwangbo, Eun Kyung Lee
- Endocrinol Metab. 2017;32(1):23-29. Published online March 20, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.1.23
- 6,269 View
- 131 Download
- 41 Web of Science
- 43 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Hyperglycemia during chemotherapy occurs in approximately 10% to 30% of patients. Glucocorticoids and L-asparaginase are well known to cause acute hyperglycemia during chemotherapy. Long-term hyperglycemia is also frequently observed, especially in patients with hematologic malignancies treated with L-asparaginase-based regimens and total body irradiation. Glucocorticoid-induced hyperglycemia often develops because of increased insulin resistance, diminished insulin secretion, and exaggerated hepatic glucose output. Screening strategies for this condition include random glucose testing, hemoglobin A1c testing, oral glucose loading, and fasting plasma glucose screens. The management of hyperglycemia starts with insulin or sulfonylurea, depending on the type, dose, and delivery of the glucocorticoid formulation. Mammalian target of rapamycin (mTOR) inhibitors are associated with a high incidence of hyperglycemia, ranging from 13% to 50%. Immunotherapy, such as anti-programmed death 1 (PD-1) antibody treatment, induces hyperglycemia with a prevalence of 0.1%. The proposed mechanism of immunotherapy-induced hyperglycemia is an autoimmune process (insulitis). Withdrawal of the PD-1 inhibitor is the primary treatment for severe hyperglycemia. The efficacy of glucocorticoid therapy is not fully established and the decision to resume PD-1 inhibitor therapy depends on the severity of the hyperglycemia. Diabetic patients should achieve optimized glycemic control before initiating treatment, and glucose levels should be monitored periodically in patients initiating mTOR inhibitor or PD-1 inhibitor therapy. With regard to hyperglycemia caused by anti-cancer therapy, frequent monitoring and proper management are important for promoting the efficacy of anti-cancer therapy and improving patients' quality of life.
-
Citations
Citations to this article as recorded by- Bridging the Gap: Pancreas Tissue Slices From Organ and Tissue Donors for the Study of Diabetes Pathogenesis
Christian M. Cohrs, Chunguang Chen, Mark A. Atkinson, Denise M. Drotar, Stephan Speier
Diabetes.2024; 73(1): 11. CrossRef - Assessment of metabolic syndrome parameters in pediatric acute lymphoblastic leukemia survivors
Ömer Kartal, Orhan Gürsel
Indian Journal of Cancer.2023; 60(3): 325. CrossRef - Increased risk of incident diabetes after therapy with immune checkpoint inhibitor compared with conventional chemotherapy: A longitudinal trajectory analysis using a tertiary care hospital database
Minyoung Lee, Kyeongseob Jeong, Yu Rang Park, Yumie Rhee
Metabolism.2023; 138: 155311. CrossRef - Analysis of the Incidence of Type 2 Diabetes, Requirement of Insulin Treatment, and Diabetes-Related Complications among Patients with Cancer
Su Jung Lee, Chulho Kim, Hyunjae Yu, Dong-Kyu Kim
Cancers.2023; 15(4): 1094. CrossRef - A Case of Pembrolizumab-Induced Diabetic Ketoacidosis and Hyperthyroidism in a Patient With Recurrent Esophageal Adenocarcinoma
Jonathan Salangsang, Surendra Sapkota, Sanjeev Kharel, Prakash Gupta, Abhishek Kalla
Cureus.2023;[Epub] CrossRef - Link between Blood Cell-Associated Inflammatory Indices and Chemotherapy-Induced Hyperglycemia in Women Affected with Breast Cancer: Clinical Studies
Krishna Prasad, Suresh Rao, Sanath Kumar Hegde, Thomas George, Rhea Katherine D'souza, Sucharitha Suresh, Manjeshwar Shrinath Baliga
South Asian Journal of Cancer.2023; 12(02): 118. CrossRef - Insulin resistance in patients with cancer: a systematic review and meta-analysis
Joan M. Màrmol, Michala Carlsson, Steffen H. Raun, Mia K. Grand, Jonas Sørensen, Louise Lang Lehrskov, Erik A. Richter, Ole Norgaard, Lykke Sylow
Acta Oncologica.2023; 62(4): 364. CrossRef - Metabolic obesity phenotypes and obesity‐related cancer risk in the National Health and Nutrition Examination Survey
Maci Winn, Prasoona Karra, Heinz Freisling, Marc J. Gunter, Benjamin Haaland, Michelle L. Litchman, Jennifer A. Doherty, Mary C. Playdon, Sheetal Hardikar
Endocrinology, Diabetes & Metabolism.2023;[Epub] CrossRef - Effects of Vitamin Intake on Blood Glucose in Cancer Patients Undergoing Chemotherapy: Quantitative and Descriptive Research
Ji Yeong Kim, Kyung Hee Lim
Korean Journal of Adult Nursing.2023; 35(2): 148. CrossRef - Oncologists’ responsibility, comfort, and knowledge managing hyperglycemia in patients with cancer undergoing chemotherapy: a cross sectional study
Teresa M. Salgado, Rotana M. Radwan, Erin Hickey Zacholski, Emily Mackler, Tonya M. Buffington, Kerri T. Musselman, William J. Irvin, Jennifer M. Perkins, Trang N. Le, Dave L. Dixon, Karen B. Farris, Vanessa B. Sheppard, Resa M. Jones
Supportive Care in Cancer.2023;[Epub] CrossRef - Diabetes management in cancer patients. An Italian Association of Medical Oncology, Italian Association of Medical Diabetologists, Italian Society of Diabetology, Italian Society of Endocrinology and Italian Society of Pharmacology multidisciplinary conse
N. Silvestris, T. Franchina, M. Gallo, A. Argentiero, A. Avogaro, G. Cirino, A. Colao, R. Danesi, G. Di Cianni, S. D’Oronzo, A. Faggiano, S. Fogli, D. Giuffrida, S. Gori, N. Marrano, R. Mazzilli, M. Monami, M. Montagnani, L. Morviducci, A. Natalicchio, A.
ESMO Open.2023; 8(6): 102062. CrossRef - Prognostic Factors for Hyperglycemia in Patients Receiving Chemotherapy
Jiyeong Kim, Kyung Hee Lim
Cancer Nursing.2023;[Epub] CrossRef - Severe Insulin Resistance in a Patient Treated With Nivolumab and Brentuximab-Vedotin for Hodgkin Lymphoma
Elif Tama, Meghan Black, Muhamad Alhaj Moustafa, Maria D Hurtado
JCEM Case Reports.2023;[Epub] CrossRef - A New Hypothesis Describing the Pathogenesis of Oral Mucosal Injury Associated with the Mammalian Target of Rapamycin (mTOR) Inhibitors
Stephen T. Sonis, Alessandro Villa
Cancers.2023; 16(1): 68. CrossRef - Management of Phosphatidylinositol-3-Kinase Inhibitor-Associated Hyperglycemia
Marcus D. Goncalves, Azeez Farooki
Integrative Cancer Therapies.2022; 21: 153473542110731. CrossRef - Metformin Induced Cognitive Impairment and Neuroinflammation in CMF-Treated Rats
Ahmad H. Alhowai, Yasser Almogbel, Ahmed A.H. Abdel, Maha A. Aldubay, Hani A. Alfheeaid, Shatha G. Felemban, Sridevi Chigurupat, Ibrahim F. Alharbi, Hindi S. Alharbi
International Journal of Pharmacology.2022; 18(2): 228. CrossRef - Glucose Influences the Response of Glioblastoma Cells to Temozolomide and Dexamethasone
Anna M Bielecka-Wajdman, Tomasz Ludyga, Daria Smyk, Wojciech Smyk, Magdalena Mularska, Patrycja Świderek, Wojciech Majewski, Christina Susanne Mullins, Michael Linnebacher, Ewa Obuchowicz
Cancer Control.2022; 29: 107327482210754. CrossRef - Cardiometabolic Comorbidities in Cancer Survivors
Leah L. Zullig, Anthony D. Sung, Michel G. Khouri, Shelley Jazowski, Nishant P. Shah, Andrea Sitlinger, Dan V. Blalock, Colette Whitney, Robin Kikuchi, Hayden B. Bosworth, Matthew J. Crowley, Karen M. Goldstein, Igor Klem, Kevin C. Oeffinger, Susan Dent
JACC: CardioOncology.2022; 4(2): 149. CrossRef - Glucose deprivation reduces proliferation and motility, and enhances the anti-proliferative effects of paclitaxel and doxorubicin in breast cell lines in vitro
Maitham A. Khajah, Sarah Khushaish, Yunus A. Luqmani, Yi-Hsien Hsieh
PLOS ONE.2022; 17(8): e0272449. CrossRef - Gemigliptin exerts protective effects against doxorubicin-induced hepatotoxicity by inhibiting apoptosis via the regulation of fibroblast growth factor 21 expression
Kyeong-Min Lee, Yeo Jin Hwang, Gwon-Soo Jung
Biochemical and Biophysical Research Communications.2022; 626: 135. CrossRef - Glucocorticoid-Induced Hyperglycemia in Oncologic Outpatients: A Narrative Review Using the Quadruple Aim Framework
Ihab Kandil, Erin Keely
Canadian Journal of Diabetes.2022; 46(7): 730. CrossRef - Real-World Experience of Monitoring Practice of Endocrinopathies Associated with the Use of Novel Targeted Therapies among Patients with Solid Tumors
Atika AlHarbi, Majed Alshamrani, Mansoor Khan, Abdelmajid Alnatsheh, Mohammed Aseeri
Medical Sciences.2022; 10(4): 65. CrossRef - The Assessment of the Hypothalamic-Pituitary-Adrenal Axis After Oncological Treatment in Pediatric Patients with Acute Lymphoblastic Leukemia
Barbara Hull, Anna Wedrychowicz, Magdalena Ossowska, Aleksandra Furtak, Joanna Badacz, Szymon Skoczeń, Jerzy B. Starzyk
Journal of Clinical Research in Pediatric Endocrinology.2022; 14(4): 393. CrossRef - Targeting Mitochondria and Oxidative Stress in Cancer- and Chemotherapy-Induced Muscle Wasting
Joshua R. Huot, Dryden Baumfalk, Aridai Resendiz, Andrea Bonetto, Ashley J. Smuder, Fabio Penna
Antioxidants & Redox Signaling.2022;[Epub] CrossRef - Onkodiabetológia III.
Róbert János Bánhegyi, Blanka Veréb, Andrea Gazdag, Beatrix Rácz, Róbert Wagner, Norbert Fülöp, Béla Pikó
Orvosi Hetilap.2022; 163(41): 1614. CrossRef - Economic burden of diabetes among medicare beneficiaries with cancer
Cassidi C McDaniel, F Ellen Loh, Devan M Rockwell, Courtney P McDonald, Chiahung Chou
Journal of Pharmaceutical Health Services Research.2021; 12(2): 142. CrossRef - The prognostic outcome of ‘type 2 diabetes mellitus and breast cancer’ association pivots on hypoxia-hyperglycemia axis
Ilhaam Ayaz Durrani, Attya Bhatti, Peter John
Cancer Cell International.2021;[Epub] CrossRef - Increased risk of diabetes in cancer survivors: a pooled analysis of 13 population-based cohort studies
Y. Xiao, H. Wang, Y. Tang, J. Yan, L. Cao, Z. Chen, Z. Shao, Z. Mei, Z. Jiang
ESMO Open.2021; 6(4): 100218. CrossRef - Sublethal concentrations of high glucose prolong mitotic arrest in a spindle assembly checkpoint activity dependent manner in budding yeast
Pinar B. Thomas, Elif E. Cavusoglu, Nur Kaluc
Biologia.2021; 76(12): 3883. CrossRef - Hyperglycemia in Childhood Acute Lymphoblastic Leukemia During Induction Chemotherapy
Nengcy Erlina Tasik Rerung, Andi Cahyadi, Nur Rochmah, Maria Christina Shanty Larasati, Mia Ratwita Andarsini, Muhammad Faizi, IDG Ugrasena, Bambang Permono
MEDICINUS.2021; 34(1): 18. CrossRef - Effect of Acute Chemotherapy on Glucose Levels in Rats
Ahmad H. Alhowail, Gena S. Alfawzan, Maha A. Aldubayan, Lolwah S. Alsalam
International Journal of Pharmacology.2020; 16(3): 276. CrossRef - Predictors of Disease Progression or Performance Status Decline in Patients Undergoing Neoadjuvant Therapy for Localized Pancreatic Head Adenocarcinoma
Alessandro Paniccia, Ana L. Gleisner, Mazen S. Zenati, Amr I. Al Abbas, Jae Pil Jung, Nathan Bahary, Kenneth K. W. Lee, David Bartlett, Melissa E. Hogg, Herbert J. Zeh, Amer H. Zureikat
Annals of Surgical Oncology.2020; 27(8): 2961. CrossRef - Fasting to enhance Cancer treatment in models: the next steps
Jing Zhang, Yanlin Deng, Bee Luan Khoo
Journal of Biomedical Science.2020;[Epub] CrossRef - Concurrent diabetic ketoacidosis and pancreatitis in Paediatric acute lymphoblastic leukemia receiving L-asparaginase
Patel Zeeshan Jameel, Sham Lohiya, Amol Dongre, Sachin Damke, Bhavana B. Lakhkar
BMC Pediatrics.2020;[Epub] CrossRef - Hyperglycemia during Adjuvant Chemotherapy as a Prognostic Factor in Breast Cancer Patients without Diabetes
Ha Rim Ahn, Sang Yull Kang, Hyun Jo Youn, Sung Hoo Jung
Journal of Breast Cancer.2020; 23(4): 398. CrossRef - Side effects of adjuvant chemotherapy and their impact on outcome in elderly breast cancer patients: a cohort study
Valentina Zanuso, Vittorio Fregoni, Lorenzo Gervaso
Future Science OA.2020; : FSO617. CrossRef - Incidence of Hyperglycemia/Secondary Diabetes in Women who have Undergone Curative Chemotherapy for Breast Cancer: First Study from India
Suresh Rao, Krishna Prasad, Soniya Abraham, Thomas George, Supreeth Kakkaje Chandran, Manjeshwar Shrinath Baliga
South Asian Journal of Cancer.2020; 09(03): 130. CrossRef - Hyperglycemic ADR Distribution of Doxorubicin From VigiBase
Jincheng Yang, Jun Yang
American Journal of Therapeutics.2019; 26(3): e428. CrossRef - Hyperglycemia During Childhood Cancer Therapy: Incidence, Implications, and Impact on Outcomes
Allison Grimes, Ashraf Mohamed, Jenna Sopfe, Rachel Hill, Jane Lynch
JNCI Monographs.2019; 2019(54): 132. CrossRef - Steroid-induced diabetes in cancer patients
Gemma Dinn
Journal of Prescribing Practice.2019; 1(12): 610. CrossRef - Risk Factors for Doxorubicin-Induced Serious Hyperglycaemia-Related Adverse Drug Reactions
Jincheng Yang, Yu Wang, Kang Liu, Wen Yang, Jianying Zhang
Diabetes Therapy.2019; 10(5): 1949. CrossRef - Effect of glucose and palmitate environment on proliferation and migration of PC3‐prostate cancer cells
Lívia Prometti Rezende, Maria Raquel Unterkircher Galheigo, Breno Costa Landim, Amanda Rodrigues Cruz, Françoise Vasconcelos Botelho, Renata Graciele Zanon, Rejane Maira Góes, Daniele Lisboa Ribeiro
Cell Biology International.2019; 43(4): 373. CrossRef - Incidence of Diabetes After Cancer Development
Yul Hwangbo, Danbee Kang, Minwoong Kang, Saemina Kim, Eun Kyung Lee, Young Ae Kim, Yoon Jung Chang, Kui Son Choi, So-Youn Jung, Sang Myung Woo, Jin Seok Ahn, Sung Hoon Sim, Yun Soo Hong, Roberto Pastor-Barriuso, Eliseo Guallar, Eun Sook Lee, Sun-Young Kon
JAMA Oncology.2018; 4(8): 1099. CrossRef
- Bridging the Gap: Pancreas Tissue Slices From Organ and Tissue Donors for the Study of Diabetes Pathogenesis

- Thyroid
- Letter: Expression of Glucagon-Like Peptide-1 Receptor in Papillary Thyroid Carcinoma and Its Clinicopathologic Significance (Endocrinol Metab 2014;29:536-44, Min Jung Jung et al.)
- Daeyoon Park, Eun Kyung Lee
- Endocrinol Metab. 2015;30(2):231-232. Published online June 30, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.2.231
- 2,611 View
- 25 Download


 KES
KES

 First
First Prev
Prev



